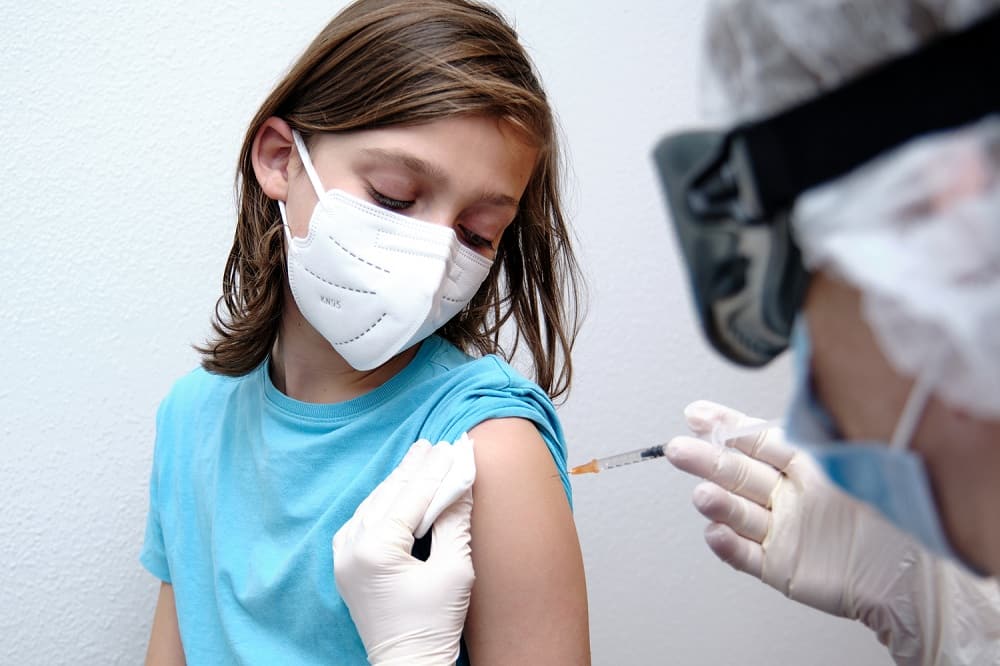
Pfizer Inc. and BioNTech SE announced that the U.S. FDA has expanded the Emergency Use Authorization (EUA) for their COVID-19 vaccine to include individuals 12 to 15 years of age. This is the first COVID-19 vaccine authorized in the U.S. for use in this age group.
“Today’s expansion of our EUA represents a significant step forward in helping the U.S. government broaden its vaccination program and help protect adolescents before the start of the next school year,” said Albert Bourla, Chairman and Chief Executive Officer, Pfizer. “We are grateful to all of our clinical trial volunteers and their families, whose courage helped make this milestone possible. Together, we hope to help bring a sense of normalcy back to young people across the country and around the world.”
The FDA based its decision on data from a pivotal Phase 3 clinical trial, which enrolled 2,260 participants aged 12 to 15 years. Topline results from this trial, announced on March 31, 2021, showed a vaccine efficacy of 100% in participants with or without prior SARS-CoV-2 infection and robust antibody responses. In the trial, the vaccine was also generally well tolerated. Participants will continue to be monitored for long-term protection and safety for an additional two years after their second dose.
As a next step following today’s FDA decision, the U.S. Centers for Disease Control and Prevention’s (CDC) Advisory Committee on Immunization Practices (ACIP) will meet to discuss recommendations for use of the Pfizer-BioNTech COVID-19 Vaccine in adolescents 12 to 15 years of age based on the amended EUA.
“Since securing the EUA in December for individuals 16 years and older, we have been working tirelessly to get our COVID-19 vaccine authorized around the world so that governments can provide it to as many people as possible,” said Ugur Sahin, M.D., CEO and Co-founder of BioNTech. “Our work is not yet complete, as we continue our research into the use of our vaccine in pediatric populations. Our goal is to submit data for pre-school and school-age children in September.”
Pfizer and BioNTech have submitted the data in adolescents 12 to 15 years of age for scientific peer review for potential publication. The data also have been submitted to other regulators around the world, including the European Medicines Agency (EMA).
In addition, the pediatric study evaluating the safety and efficacy of the Pfizer-BioNTech COVID-19 Vaccine in children 6 months to 11 years of age is ongoing. Pfizer and BioNTech expect to have definitive readouts and, subject to the data generated, submit for an EUA or a variation to Conditional Marketing Authorizations for two cohorts, including children 2-5 years of age and 5-11 years of age, in September. The readout and submission for the cohort of children 6 months to 2 years of age are expected in the fourth quarter.
The Pfizer-BioNTech COVID-19 Vaccine, which is based on BioNTech proprietary mRNA technology, was developed by both BioNTech and Pfizer. BioNTech is the Marketing Authorization Holder in the European Union, and the holder of emergency use authorizations or equivalents in the United States (together with Pfizer), United Kingdom, Canada and other countries in advance of a planned application for full marketing authorizations in these countries.
The Pfizer-BioNTech COVID-19 Vaccine has not been approved or licensed by the U.S. Food and Drug Administration (FDA), but has been authorized for emergency use by FDA under an Emergency Use Authorization (EUA) to prevent Coronavirus Disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for use in individuals 12 years of age and older. The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of the medical product under Section 564 (b) (1) of the FD&C Act unless the declaration is terminated or authorization revoked sooner.
About Pfizer: Breakthroughs That Change Patients’ Lives
At Pfizer, we apply science and our global resources to bring therapies to people that extend and significantly improve their lives. We strive to set the standard for quality, safety and value in the discovery, development and manufacture of health care products, including innovative medicines and vaccines. Every day, Pfizer colleagues work across developed and emerging markets to advance wellness, prevention, treatments and cures that challenge the most feared diseases of our time. Consistent with our responsibility as one of the world’s premier innovative biopharmaceutical companies, we collaborate with health care providers, governments and local communities to support and expand access to reliable, affordable health care around the world. For more than 170 years, we have worked to make a difference for all who rely on us.