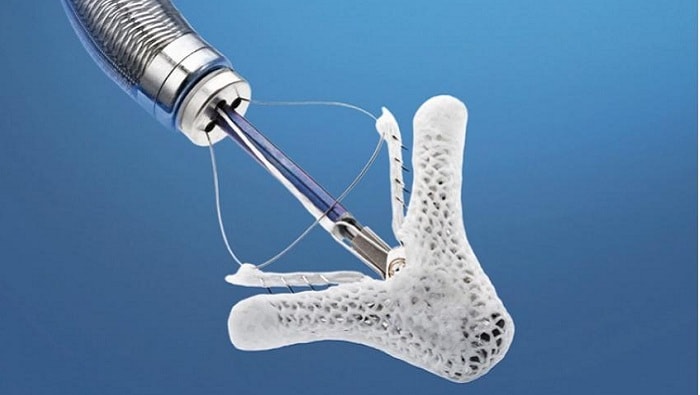
Abbott announced that Japan’s Ministry of Health, Labour and Welfare (MHLW) has approved the company’s MitraClip device for treatment of people with mitral regurgitation (MR), a serious, progressive heart disease in which the mitral valve does not close properly, allowing blood to flow backward into the heart.
MR causes many life-altering symptoms, and left untreated, can ultimately lead to heart failure and death. i,ii,iii Most people affected are elderly as MR incidence increases with age.iv
“MitraClip provides a new treatment option for many people with severe mitral regurgitation who cannot undergo the mitral valve surgery,” said Kentaro Hayashida, M.D., Cardiovascular Internal Medicine, Keio University, Tokyo and principal investigator of the AVJ-514 clinical trial. “The introduction of MitraClip therapy in Japan will help more people who previously had limited options return to better health faster, with dramatically reduced symptoms.”
Currently, the standard-of-care treatments for mitral regurgitation in Japan are limited to open-heart surgery and medication. Surgery is not a viable option for some patients because of advanced age or other comorbidities, and medications typically only mask symptoms, instead of treating the underlying issue of the valve itself.
“It is our mission to help people suffering from structural heart disease return to better health and quality-of-life by providing technologies and therapies that advance how people are treated,” said Michael Dale, vice president for Abbott’s structural heart business. “With the approval of our revolutionary MitraClip device in Japan, we can help more people live better by reducing the severity of their extremely life-altering illness in a safe and predictable way.”
The MitraClip system is a catheter-based, minimally-invasive therapy that is delivered to the heart through a blood vessel in the leg. By securing a portion of the leaflets of the mitral valve with a clip, the heart can pump blood more efficiently throughout the body, thereby relieving the symptoms of severe MR and improving patient quality of life. v
After obtaining CE mark approval in Europe in 2008, MitraClip was approved by the U.S. Food & Drug Administration (FDA) in 2013. To date, more than 50,000 people have been treated by MitraClip in nearly 50 countries. With approval in Japan, the device will be indicated to treat both severe degenerative mitral regurgitation (DMR) and functional mitral regurgitation (FMR) heart diseases.
About the AVJ-514 Trial
The approval in Japan is based on the results of the AVJ-514 clinical trial and extensive global experience and clinical data available for the MitraClip System. In the AVJ-514 study, patients with severe degenerative mitral regurgitation or functional mitral regurgitation were treated at six facilities in Japan. Enrolled patients were limited to those who were not candidates for mitral valve surgery. In a late breaking session at the 81st Annual Scientific Meeting of the Japanese Circulation Society in March 2017, the results of 30-day follow-up observation after the procedure in the clinical trial were reported: at 30 days, 86.7 percent of patients had MR ≤2+ and 96.7 percent were NYHA class I/II. There were no major adverse events (death, stroke, myocardial infarction, renal failure, non-elective cardiovascular surgery) in the study through 30 days.
About Degenerative and Functional Mitral Regurgitation
Degenerative mitral regurgitation (DMR) is caused by abnormality of valve structures including the valve leaflets, valve ring, and chordae tendineae. Functional mitral regurgitation (FMR) is a disease that occurs when the left ventricle of the heart dilates, leading to incomplete coaptation of the mitral valve.
About Abbott
At Abbott, we’re committed to helping people live their best possible life through the power of health. For more than 125 years, we’ve brought new products and technologies to the world — in nutrition, diagnostics, medical devices and branded generic pharmaceuticals — that create more possibilities for more people at all stages of life. Today, 94,000 of us are working to help people live not just longer, but better, in the more than 150 countries we serve. Connect with us at www.abbott.com
For further information:
Abbott Japan Media:
Toshiyuki Shimizu,
03-4555-1002,
Abbott US Media:
Mary Kokkinen,
(408) 845-0808,
Abbott Financial:
Mike Comilla,
(224) 668-1872