This funding represents the international support for R&D, particularly to promote the acceleration and utilisation of artificial intelligence in clinical decision making.
My Personal Therapeutics, a London-based medtech/digital therapeutics company and Pentavere Research Group, a Canadian-based Artificial Intelligence and data insight company announced their successful EUREKA Collaborative R&D: AI and Quantum Technologies Competition award for project titled “Utilisation of AI to develop Personalised Treatment Plans for cancer”. Funding totalling £792,000* is co-funded by the UK’s innovation agency, Innovate UK, and Canada’s National Research Council’s Industrial Research Assistant Program, as part of their Collaborate R&D programme.
These funds will be used by Pentavere Research Group and My Personal Therapeutics to access Genomics England’s whole genome sequencing lung cancer data set and selectively generate drosophila avatars for high-throughput drug screening. The resulting tumour genomic profile and corresponding drug treatment recommendation data will feed into our AI Personal Discovery Process predictive model. Potentially, some of the funding can support personalised treatment recommendations for lung cancer patients in the UK.
“This Eureka award will partly fund this ground-breaking collaboration between My Personal Therapeutics, Pentavere and Genomics England towards the development of our rapid personalised cancer therapeutics offering – AI PDP” shared My Personal Therapeutics’ CEO Laura Towart.
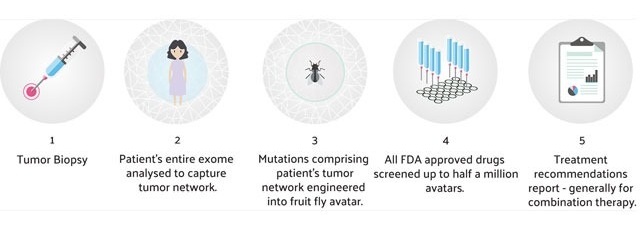
My Personal Therapeutics is a London based medtech/digital therapeutics company offering personalised cancer therapeutics utilising technology developed at Mt Sinai Medical Center. The Personal Discovery Process technology leverages Big Data curated from electronic health records, and genomics to build personalised fruit fly ‘avatars’ that model individual patients at an unprecedented level of complexity. Using robotics, thousands of drugs are screened in combinations to identify drug cocktails designed to target the tumour while preserving the patient’s quality of life. Nearly all combinations incorporate non-cancer drugs, making them less toxic and more affordable. We are integrating AI/ predictive modeling to enable rapid personalised treatment recommendations.
*Exact amount / contributions:
Innovate UK: £330,553
NRC IRAP: £141,329
Pentavere Research Group: £180,306
My Personal Therapeutics: £140,259
Total: £792,447
Contact Details
My Personal Therapeutics
Laura Towart, Chief Executive Officer
Pentavere:
Aaron Leibtag, Chief Executive Officer
About My Personal Therapeutics
My Personal Therapeutics, a London based biotechnology/digital health company offers personalised cancer therapeutics utilizing comprehensive, genomics-based drug screening technology developed at and commercialised in partnership with the Icahn School of medicine at Mt Sinai Medical Center.
The Personal Discovery Process is effectively a massive clinical trial for a single patient. Scientists at My Personal Therapeutics engineer the genetic complexity of each patient’s unique tumour network into an army of 400,000 fruit fly “avatars.” Using robotics, up to 2000 FDA approved drugs including non-cancer drugs are evaluated, to identify drug combinations designed to treat the tumour itself. My Personal Therapeutics is integrating predictive modeling and AI to enable rapid and affordable treatment recommendations.
About Pentavere Research Group
Pentavere is one of Canada’s fastest-growing healthcare technology companies. Based in Toronto Canada, we are transforming how clinical data is organized and applied in the healthcare sector. Hospitals, health networks, researchers and pharmaceutical companies use Pentavere’s powerful data curation and insight engine, DARWEN, to harness the full potential of their data to improve patient outcomes. We combine Artificial Intelligence (AI) technologies with clinical expertise to create lifesaving insights and real-world evidence that improve care for patients – at a speed and accuracy few thought was possible. In short, we find the needles in the haystack that save lives. By unlocking this valuable information, Pentavere is making a real impact on human health.
About EUREKA
EUREKA is a publicly-funded, intergovernmental network, involving over 40 countries. EUREKA’s aim is to enhance European competitiveness by fostering innovation-driven entrepreneurship in Europe, between small and large industry, research institutes and universities. By doing this, EUREKA concentrates the existing potential of experts, of knowledge, research facilities and financial resources in a more efficient way. EUREKA is constantly proving its value through a wealth of success stories – innovative products, processes and services that have been launched onto the market over the last 30 years, creating additional turnover and jobs for European companies, small and large – and by supporting the internationalization of businesses with innovative ideas.
EUREKA is a leading open platform for international cooperation in innovation. It remains to this day the only initiative of its kind committed to the ‘bottom-up’ principle – ensuring that any R&D project with a good business plan receives the support it deserves, independent of its technological nature, or the type of organisations involved.
About Innovate UK
Innovate UK drives productivity and economic growth by supporting businesses to develop and realise the potential of new ideas.
We connect businesses to the partners, customers and investors that can help them turn ideas into commercially successful products and services and business growth.
We fund business and research collaborations to accelerate innovation and drive business investment into R&D. Our support is available to businesses across all economic sectors, value chains and UK regions.
About National Research Council of Canada Industrial Research Assistant Program (NRC-IRAP)
The National Research Council of Canada Industrial Research Assistance Program (NRC IRAP) provides advice, connections, and funding to help Canadian small and medium-sized businesses increase their innovation capacity and take ideas to market.
The NRC IRAP’s mission is to have an impact by advancing knowledge, applying leading-edge technologies, and working with other innovators to find creative, relevant and sustainable solutions to Canada’s and the world’s current and future economic, social and environmental challenges.



















