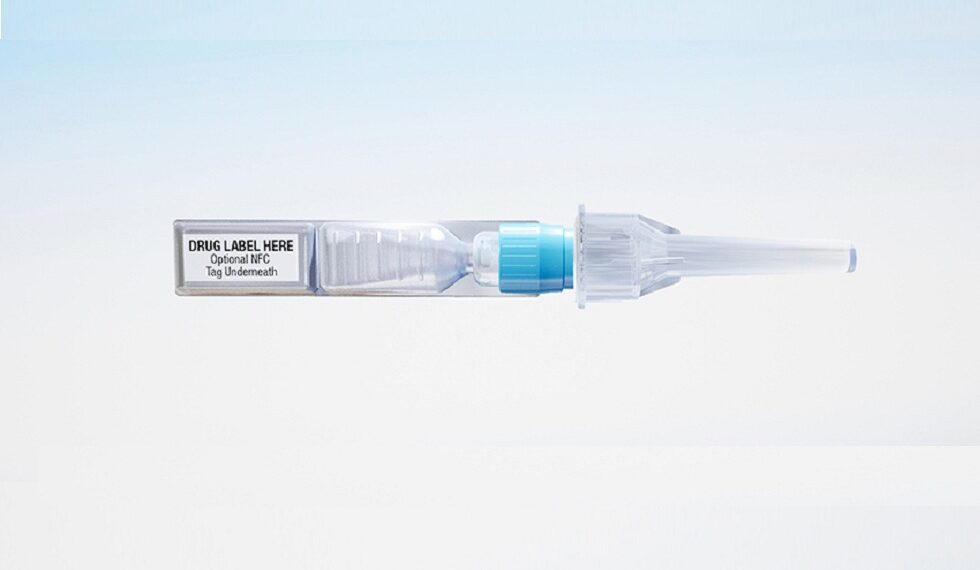
Fareva and ApiJect Systems, Corp. announced a 10-year licensing agreement to install three Blow-Fill-Seal production lines that when operational will be able to fill-finish more than 500 million doses per year of vaccines and other large molecule injectable drugs using ApiJect’s innovative prefilled injector technology. Fareva intends to invest more than €50M (US$ 56.5M) for these three manufacturing lines, with support from the Government of France, through the “France Relance” industry initiative and the Investments for the Future Program (PIA).
The three manufacturing lines and ApiJect’s technology utilize the Blow-Fill-Seal (BFS) aseptic packaging process. BFS has demonstrated its ability to package certain vaccines and large molecule formulations, and ApiJect is helping to expand BFS’s capabilities to additional drug products, including temperature sensitive pharmaceuticals. When cleared by regulators, ApiJect will provide the necessary needle hub and other attachable components to convert finished BFS single-dose containers from these lines into easy-to-assemble, ready-to-use prefilled injectors.
COVID-19 has demonstrated the strategic importance of regional pharmaceutical manufacturing to ensure strong and reliable medical supply chains. By investing in these three BFS lines and installing ApiJect technology, Fareva is helping to increase Europe’s high-speed, high-volume fill and finish capacity by potentially more than 500 million doses per year. And, the compact polyethylene-based supply chain of BFS helps establish and maintain dependable and resilient fill-finish capacity, even in the face of additional COVID-19 pandemic waves and their supply chain repercussions.
ApiJect CEO and Chair Jay Walker commented: “This commitment by Fareva, with the support of the French Government, to expand fill-finish capacity is the right step at the right time. We are proud to partner with Fareva in bringing the next generation of injection technology to global health.”
Mr. Walker continued: “Our mission at ApiJect is to support and execute a strategy of expanded distributed fill-finish capacity in the U.S. and regionally around the world. This is desperately needed in the battle against COVID, particularly as we continue to face emerging variants. Distributed fill-finish capacity will also yield huge dividends when future pandemics and other possible bio-emergencies threaten our populations. Fareva, along with its support from the French Government, is showing itself to be a vital leader in confronting and defeating the COVID threat.”
The new lines will be situated in Fareva Excelvision, located in Annonay, France, which has more than 50 years of experience manufacturing BFS containers and exporting products around the world. The BFS machines will be purchased from Rommelag in Germany. Clean rooms for these new lines will be operated in a Biosafety Level 2 (BSL2) environment, allowing them to handle most vaccines. The objective is to start the first validation batches in June 2022. In addition, this capacity expands ApiJect licensing of its BFS manufacturing and device technology to the USA and Europe.
Olivier Dussopt. Minister Delegate for Public Accounts said: “I am very happy that Fareva, and their manufacturing site of Annonay in Ardeche, invests in such a development project, concerning 3 production lines for an amount of 50 million euros. The government will support those investments with a program of 36,9 million euros. This is an excellent news for the local territory as it will create 150 jobs and this is also an excellent news for France and Europe that will benefit from this high technology for the manufacturing of pharmaceutical drugs and more precisely of vaccines.“
Agnès Pannier-Runacher, Minister Delegate for the Industry declared: “I welcome the project implemented by Fareva in collaboration with APIJect, a world premiere that will enable the production up to 500 million of injectable pharmaceutical products from 2022. This ambitious 50 million euros project will strengthen our healthcare industry in France and shows that innovation is not only in the vaccines but also stands in the injection devices. It is also the reason why we have agreed to support it with the France Relance program.”
ABOUT APIJECT SYSTEMS, CORP.
ApiJect Systems, Corp. is a public-benefit medical technology company working to bring prefilled, single-dose injections to more people in every market. The ApiJect Platform enables pharmaceutical and biotech companies to design scalable prefilled injectors and efficiently fill-finish them with their injectable drug products. This can be done either on one of their own ApiJect-licensed Blow-Fill-Seal packaging lines or at one of our world-class manufacturing partners.
ABOUT FAREVA
Fareva, a family-owned company, whose strength lies in its financial independence, is one of the world’s leading CDMOs in the pharmaceuticals, cosmetics, make-up, and industrial and homecare fields. Fareva operates in 12 countries with 42 factories and employs more than 13 000 employees with annual revenues reaching € 1.8 billion in 2020.