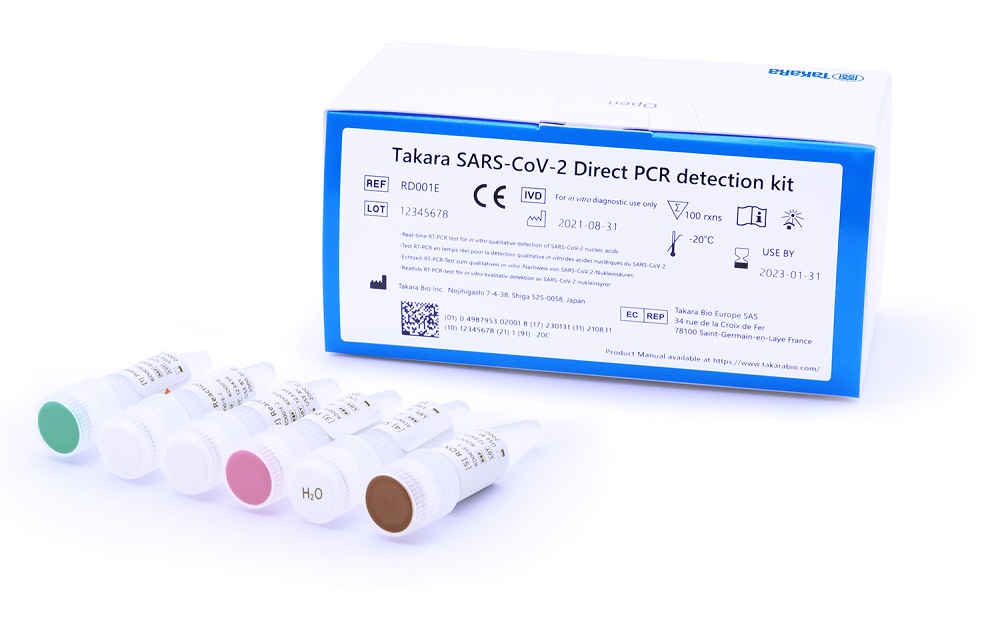
Takara Bio Europe (TBE) announced the launch of an in vitro diagnostic assay for the qualitative detection of novel coronavirus (SARS-CoV-2) from patients suspected of having Covid-19, based on real-time reverse transcriptase PCR (RT-qPCR). This CE-IVD kit complies with the Directive 98/79/EC on in vitro medical devices and is available for immediate distribution in selected countries.
The Takara SARS-CoV-2 Direct PCR detection kit, which was approved in Japan last year as an in vitro diagnostic kit, has been designed to detect SARS-CoV-2 in a simpler and faster manner. Nasopharyngeal or nasal swab samples, along with crude saliva samples, can be processed directly with this assay without RNA extraction, thus streamlining the protocol and giving a result in just 60 minutes.
This kit contains two primer/probe sets specific to the regions of the SARS-CoV-2 nucleocapsid genes (N1 and N2), based on the sequences reported by the Center for Diseases Control and Prevention (CDC)¹. It also includes a primer/probe set to detect the human RNase P (RP) gene as an internal control. The Takara SARS-CoV-2 Direct PCR detection kit uses Takara Bio’s worldwide renowned enzymes to offer reliable results that clinicians can trust. The kit’s simple yet efficient protocol has been tested and validated on commonly used thermocyclers and can accommodate a wide range of samples, making it a versatile and economical solution for all laboratories.
“Expanding our best-in-class enzymes portfolio already widely used in a broad range of diagnostic assays, to launch our first CE-IVD-marked kit is an important step for us”, says Pierre Lacaze, Managing Director at TBE. “We believe that bringing a faster, simpler, yet cost-efficient solution to market will help address current bottlenecks for diagnostic labs across Europe. The reliability and convenience of this kit has already convinced clinicians in Japan, where it has been used for millions of SARS-CoV-2 tests. Today, we are pleased to serve our European customers as well.”
With this kit, TBE is proud to support governments, medical staff and scientists in their fight against the Covid-19 pandemic, and to contribute to developing innovative solutions for the diagnosis of respiratory diseases.
About Takara Bio Europe
Takara Bio Europe is a wholly owned subsidiary of Takara Bio Inc., who develops, manufactures, and distributes a wide range of life science reagents under the Takara, Clontech, and Cellartis brands.
Key products include high-performance qPCR and PCR reagents (including the Takara Ex Taq®, Takara LA Taq®, Titanium®, and Advantage® enzymes), Cellartis stem cells and stem cell reagents, RT enzymes and SMART library construction kits, the innovative In-Fusion® cloning system, Guide-it gene editing tools, Tet-based inducible gene expression systems, and Living Colors® fluorescent proteins. Takara Bio’s portfolio supports applications including NGS, gene discovery, regulation, and function studies, as well as genetic analysis, protein expression and purification, gene editing, stem cell studies, and plant and food research.
About Takara Bio Inc.
Takara Bio Inc., a world leader in biotechnology research and development, offers a host of life science research solutions, from enzymes and GMP-grade reagents to contracted cell and gene therapy manufacturing services and is the developer of the RetroNectin reagent, a world standard in gene therapy protocols. Takara Bio is committed to preventing disease and improving the quality of life for all people through the use of biotechnology.