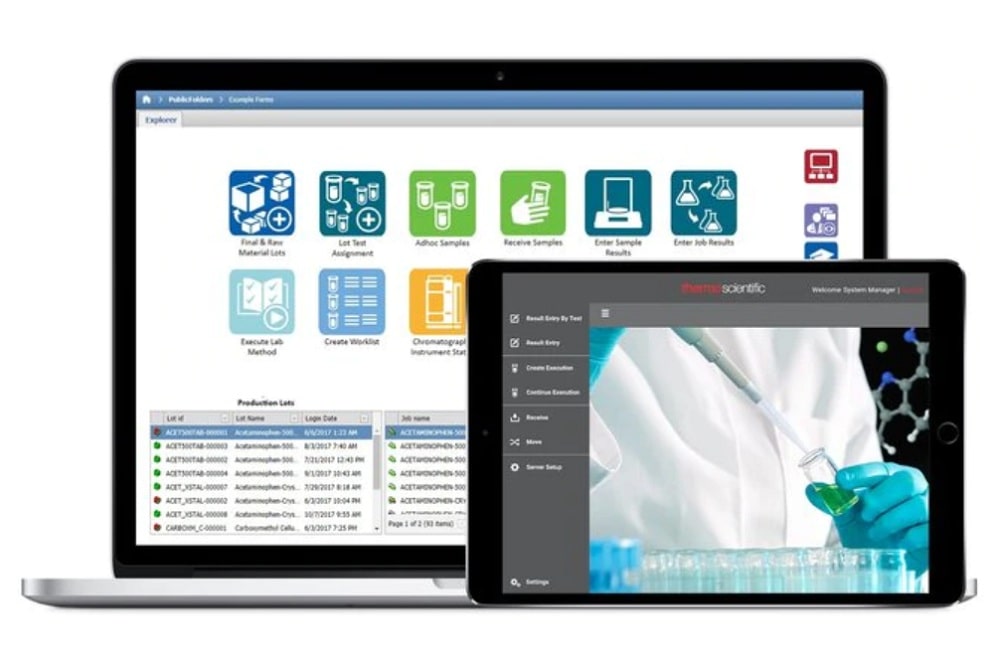
In response to the data management needs of manufacturing QA/QC and contract laboratories, the latest version of SampleManager Laboratory Information Management System (LIMS) software offers an advanced solution: a built-in electronic lab notebook (ELN), capabilities to streamline operations within contract laboratories and enhanced dashboard configuration for tailored data visualization.
SampleManager LIMS software 12.3 features a fully integrated ELN, simplifying storage, search and retrieval of supplementary information such as unstructured data. As a result, the need for time and resource intensive rework caused by disparate, incomplete and unsearchable data is eliminated, while facilitating improved connectivity and collaboration between laboratories. The ELN adds to the already rich capabilities of the SampleManager LIMS software suite, integrating with the existing LIMS, lab execution system (LES) and scientific data management system (SDMS). Furthermore, the software maximizes process efficiencies within contract laboratories, providing a single system to easily manage customers’ work, streamline administrative tasks including pricing and invoicing, and provide customers with a secure, professional portal to log requests and access results.
Importantly, SampleManager LIMS software 12.3 does not require specialized IT personnel to set up dashboards. Instead, the software offers scientists and researchers the option to configure dashboards themselves, to provide user or role views of key performance indicators (KPIs) or other relevant information, allowing for faster, more informed decision making.
“Building on more than 30 years of laboratory informatics knowledge and technology expertise, SampleManager LIMS software continues to provide rich functionality and put control in the hands of users to drive efficiencies, connect data and deliver actionable information,” said Dr. Cheryl Moody Bartel, head of product management for digital science, Thermo Fisher Scientific. “The new version of the software introduces further capability and usability enhancements to enable manufacturing QA/QC and contract laboratories to improve management of their laboratory operations and drive further insights from their data.”
One of the most widely deployed LIMS in the world, SampleManager LIMS software delivers laboratory management, data management and process execution capabilities all in a single solution. The software integrates with instruments and equipment, such as Manufacturing Execution Systems (MES), Process Information Management Systems (PIMS), Enterprise Resource Planning (ERP) systems and Chromatography Data Systems (CDS), enabling a holistic view of operations for quicker decision making, better process control and improved productivity. Through configuration, SampleManager LIMS software can be tailored to suit the needs of an extensive range of business operations and laboratory workflows, from smaller individual deployments through to global multi-site implementations.
About Thermo Fisher Scientific
Thermo Fisher Scientific Inc. is the world leader in serving science, with annual revenue exceeding $25 billion. Our Mission is to enable our customers to make the world healthier, cleaner and safer. Whether our customers are accelerating life sciences research, solving complex analytical challenges, improving patient diagnostics and therapies or increasing productivity in their laboratories, we are here to support them. Our global team of more than 75,000 colleagues delivers an unrivaled combination of innovative technologies, purchasing convenience and pharmaceutical services through our industry-leading brands, including Thermo Scientific, Applied Biosystems, Invitrogen, Fisher Scientific, Unity Lab Services and Patheon.