The U.S. Food and Drug Administration, FDA approves Brinsupri, Insmed’s oral drug for a lung disease, the company announced. This marks the first treatment specifically created to address this chronic lung condition.
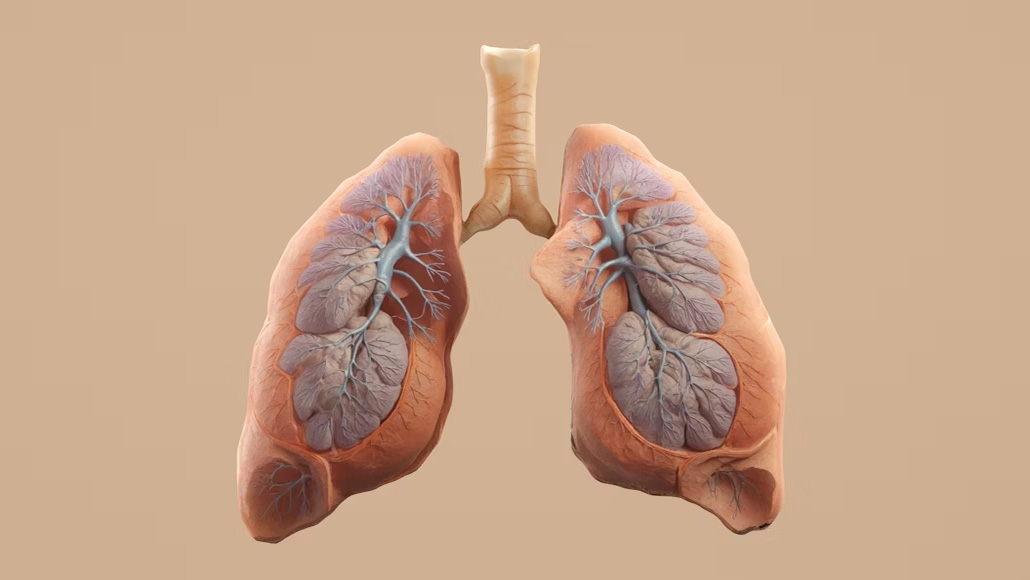
Brinsupri, the drug introduced by Insmed, is developed to treat non-cystic fibrosis bronchiectasis. In this chronic lung condition, the airways sustain permanent damage, which leads to persistent coughing and excessive mucus production.
The American Lung Association estimates that between 350,000 and 500,000 adults in the United States live with this particular lung condition.
The drug Brinsupri acts by blocking certain inflammatory enzymes in the white blood cells. This prevents them from becoming overactive, which can cause lung damage. Other treatments in the past were primarily to manage the symptoms of this condition. These treatments ranged from the use of antibiotics, surgical intervention, or devices like flutter valves to aid the clearing of the airways.
Insmed’s application for approval was based on a late-stage trial involving 1,680 adults and 41 adolescent patients. The study revealed that Brinsupri significantly reduced the frequency of respiratory symptoms, especially of conditions like chronic cough.
The FDA approves Brinsupri as the first treatment for the condition, as the drug was found to be safe and tolerable at the two doses tested, one of 10 milligrams and the other of 25 milligrams.
Other treatments in development for non-cystic fibrosis bronchiectasis include AstraZeneca’s benralizumab and Zambon’s inhaled antibiotic therapy CMS I-neb.
This latest FDA approval is Insmed’s second, following 2018’s clearance of Arikayce, which was approved for treating chronic lung infections caused by bacteria commonly found in soil and water.