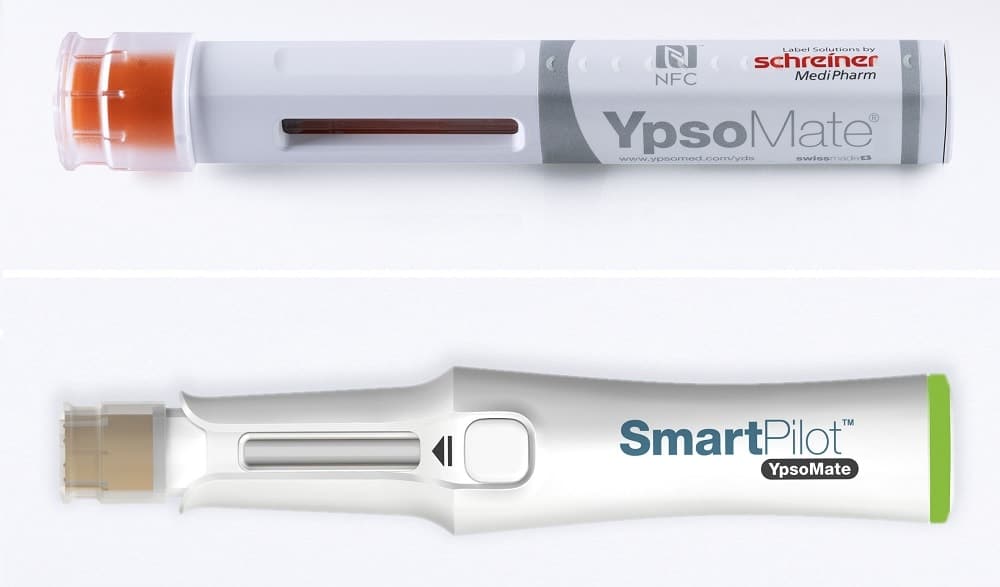
Schreiner MediPharm, a Germany-based global provider of specialty pharmaceutical labeling solutions for over 65 years, in cooperation with Swiss-based Ypsomed, has developed an NFC-Label that is applied to the YpsoMate® autoinjector.
Stored information about the medication is transmitted to its electronic add-on – the novel SmartPilot™ – and is recorded via the autoinjector and logged by the SmartPilot™. The resulting connected device enhances the safety of patients and assists them in the self-administration of medicines to better comply with prescribed therapies.
YpsoMate® is an autoinjector for self-medication of a wide range of pharmaceutical substances and in a diverse range of therapeutic areas. To turn this injection platform into a smart device, Ypsomed developed the easy-to-use SmartPilot™ – a reusable add-on with embedded sensor technology. The autoinjector is pushed into the SmartPilot™ and is automatically recognized due to the NFC-Label applied to the injection aid. The information about the medication stored on the NFC chip is transmitted via the SmartPilot™ to a related smartphone app via Bluetooth connection.
The technology behind this connected device is the interlinking of diverse sensors and the label with an integrated NFC chip, which Schreiner MediPharm adapted for YpsoMate®. The NFC chip identifies and authenticates the medicine and checks its expiration date.
According to the prescribed therapy, the patient can be guided in real time through the injection process by means of the SmartPilot™ sensors, and feedback on the correct administration of the medicine can be transmitted. It is also possible to store the entire injection history in detail — from injection time and success through to patient well-being — and to share it with other stakeholders. The attending physician, for instance, is then able to use this information to adjust the continuing therapy plan.
“The integration of NFC technology, which has to ensure reliable reading, in a very small space posed the primary challenge in this label development. Subsequent ease of processing was another specified objective,” explains Arne Rehm, Product Manager RFID/NFC Solutions at Schreiner MediPharm.
“The cooperation between Ypsomed and Schreiner MediPharm yielded an innovative and complete solution for pharmaceutical manufacturers, who are now able to provide patients with the SmartPilot™, a valuable tool supporting them in self-medication efforts,” adds Andreas Schneider, Innovation & Business Development Manager at Ypsomed.
About Ypsomed
The Ypsomed Group is a leading developer and manufacturer of injection and infusion systems for self-medication and a renowned diabetes specialist with over 30 years’ experience. As a leader in innovation and technology, Ypsomed is the preferred partner for pharmaceutical and biotech companies for the supply of pens, autoinjectors and infusion systems to administer liquid drugs. Ypsomed promotes and sells its product portfolio under the umbrella brands mylife™ Diabetescare directly to patients or through pharmacies and clinics and under YDS Ypsomed Delivery Systems as business-to-business to pharmaceutical companies. Ypsomed has its headquarters in Burgdorf, Switzerland, and operates a global network of manufacturing sites, subsidiaries and distributors. The Ypsomed Group employs almost 1,500 employees.