The pharmaceutical industry faces mounting pressure to develop high-volume injectable formulations that can deliver therapeutic doses previously restricted to intravenous administration through more convenient subcutaneous routes. These formulation challenges represent complex scientific puzzles involving viscosity management, stability optimization, and delivery system engineering that require innovative approaches to overcome traditional limitations. As biologic therapies become increasingly sophisticated and dosing requirements escalate, the need for advanced high-volume injectable formulations continues growing across diverse therapeutic areas including oncology, immunology, and rare disease treatments.
High-volume injectable formulations typically exceed traditional subcutaneous injection volumes of 1-2 milliliters, extending into ranges of 3-20 milliliters depending upon therapeutic requirements and delivery technologies. These expanded volumes enable administration of higher drug doses while maintaining patient convenience and reducing healthcare costs associated with clinical infusion procedures. However, the development of effective high-volume formulations requires addressing fundamental challenges related to drug concentration, solution properties, and injection tolerability.
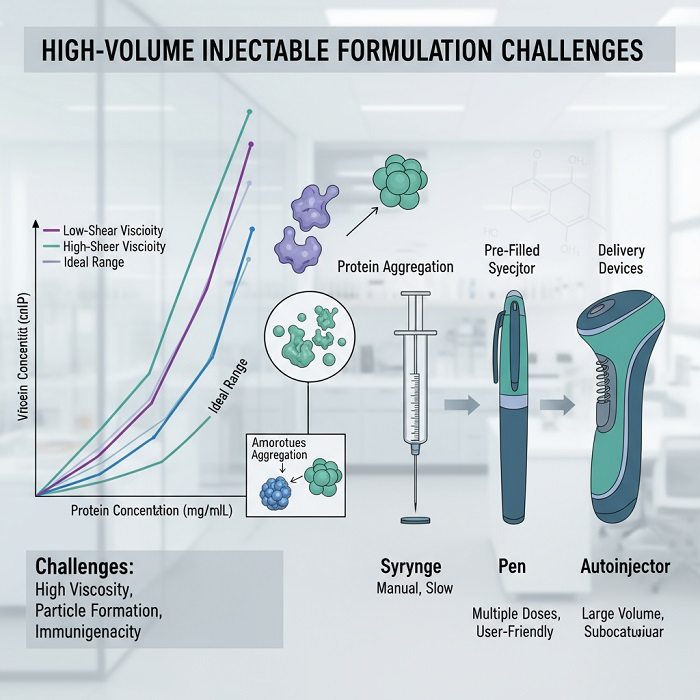
Viscosity Challenges and Formulation Strategies
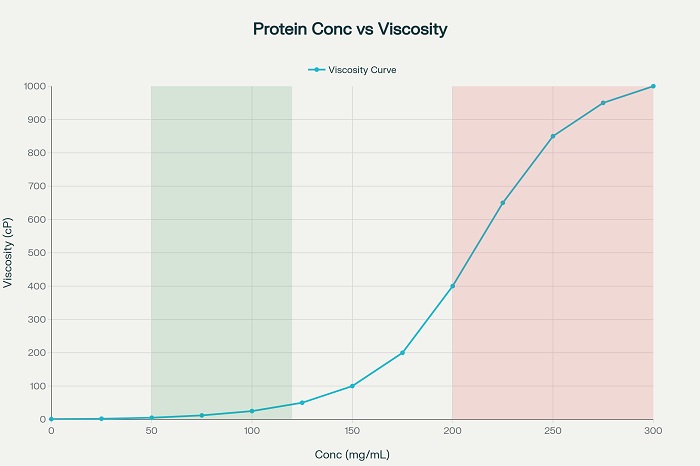
The relationship between drug concentration and solution viscosity represents one of the most significant obstacles in developing high-volume injectable formulations. Protein therapeutics demonstrate exponential increases in viscosity as concentrations rise above 100 mg/mL, creating delivery challenges that can render formulations impractical for subcutaneous administration. Advanced formulation strategies address these challenges through multiple complementary approaches that reduce viscosity while maintaining drug stability and bioactivity.
Excipient optimization utilizes specialized additives that disrupt protein-protein interactions responsible for viscosity increases. Arginine and other amino acids function as chemical chaperones that prevent aggregation and reduce intermolecular attractions. Surfactants including polysorbate 80 and poloxamer 188 provide interfacial stabilization that maintains protein structure while reducing solution viscosity. Salt optimization through ionic strength adjustments can significantly influence protein interactions and resulting viscosity characteristics.
Co-solvent systems incorporate organic solvents or polyols that modify solution properties and reduce viscosity through molecular interactions. Propylene glycol and glycerol represent commonly utilized co-solvents that demonstrate compatibility with protein therapeutics while providing viscosity reduction benefits. These systems require careful optimization to maintain protein stability while achieving desired flow characteristics for injection procedures.
pH adjustment represents another critical parameter that influences both protein stability and solution viscosity. Proteins demonstrate pH-dependent conformational changes that significantly affect intermolecular interactions and aggregation propensity. Optimal pH selection requires balancing stability, viscosity, and injection tolerability considerations while maintaining therapeutic activity throughout storage periods.
Temperature effects on solution viscosity provide opportunities for formulation optimization through controlled warming systems. Many high-viscosity formulations demonstrate significant viscosity reductions at body temperature, enabling practical injection procedures when formulations are appropriately warmed prior to administration. Advanced delivery devices incorporate warming mechanisms that optimize formulation flow properties during injection procedures.
Protein Aggregation and Stability Considerations
High-concentration formulations create environments that promote protein aggregation through increased molecular crowding effects and enhanced interaction opportunities. These aggregation processes can reduce therapeutic potency while potentially increasing immunogenicity risks through formation of particulate matter and altered protein conformations. Preventing aggregation requires comprehensive understanding of protein behavior under stress conditions and implementation of appropriate stabilization strategies.
Mechanical stress during manufacturing and handling represents a major aggregation risk factor for high-volume injectable formulations. Mixing procedures, filtration processes, and filling operations can expose proteins to shear forces that promote unfolding and aggregation. Gentle processing techniques and optimized manufacturing protocols minimize mechanical stress exposure while maintaining production efficiency and product quality.
Temperature excursions during storage and distribution create additional aggregation risks that require careful formulation optimization and supply chain management. Proteins may undergo conformational changes during temperature fluctuations that promote aggregation even after returning to appropriate storage conditions. Formulation strategies that enhance thermal stability enable expanded temperature tolerance and improved supply chain flexibility.
Oxidation represents another significant degradation pathway that can compromise high-concentration formulations through chemical modification of susceptible amino acid residues. Antioxidants including methionine, ascorbic acid, and alpha-tocopherol provide protection against oxidative degradation while maintaining compatibility with protein therapeutics. Nitrogen blanketing and oxygen-impermeable packaging systems provide additional protection against oxidative stress.
Surface interactions with container materials can promote protein adsorption and subsequent aggregation or loss of bioactivity. Silicone-free container systems and surface passivation treatments reduce protein interactions while maintaining container integrity and functionality. Advanced container materials including cyclic olefin polymers provide enhanced compatibility with high-concentration protein formulations.
Advanced Delivery Technologies and Device Innovation
Large-volume subcutaneous injection requires specialized delivery devices that can accommodate increased formulation volumes while maintaining patient comfort and injection success rates. Traditional autoinjectors designed for small volumes cannot provide sufficient force or injection duration for high-volume formulations, necessitating development of advanced delivery platforms with enhanced capabilities.
Wearable injector technologies enable extended injection procedures that gradually deliver large volumes over minutes or hours rather than seconds. These systems utilize miniaturized pumps and control electronics to provide controlled delivery rates that optimize patient comfort while ensuring complete dose administration. Battery-powered actuation systems provide consistent delivery force regardless of formulation viscosity or patient movement.
Gas-powered injection systems utilize compressed carbon dioxide or nitrogen to provide consistent actuation force throughout injection procedures. These systems maintain constant delivery pressure regardless of formulation properties, enabling reliable administration of high-viscosity solutions that would challenge spring-powered devices. Pressure regulation systems ensure patient safety while optimizing delivery performance.
Needle technology advances address challenges associated with high-viscosity formulation delivery through optimized bore sizes and surface treatments. Large-gauge needles reduce injection force requirements but may increase patient discomfort, while specialized coatings and tip geometries can improve flow characteristics without increasing needle diameter. Ultra-thin wall needle designs maximize internal diameter while minimizing external dimensions.
Multi-chamber systems enable administration of incompatible formulation components through separate injection pathways or sequential delivery procedures. These systems can accommodate drug combinations that would be unstable in single formulations while maintaining injection convenience and patient acceptance. Automated mixing capabilities enable combination therapies without requiring complex preparation procedures.
Manufacturing Process Optimization
High-volume injectable formulations require specialized manufacturing approaches that accommodate increased batch sizes while maintaining product quality and consistency. Conventional manufacturing equipment may not provide adequate capacity or mixing capabilities for large-scale production of high-concentration formulations, necessitating process modifications and equipment upgrades.
Mixing system design becomes critical for high-viscosity formulations that may require extended mixing times and specialized impeller geometries. High-shear mixing systems provide enhanced mixing efficiency but must be carefully controlled to prevent protein damage through excessive mechanical stress. Computational fluid dynamics modeling enables optimization of mixing parameters and equipment design for specific formulation requirements.
Filtration processes face significant challenges when processing high-viscosity solutions that may not flow readily through conventional membrane systems. Tangential flow filtration and other specialized techniques provide alternatives that accommodate viscous formulations while maintaining filtration efficiency and product quality. Membrane selection and system design optimization enable processing of challenging formulations without compromising sterility or purity.
Filling operations require specialized equipment and procedures that can handle viscous formulations accurately and consistently. Positive displacement filling systems provide enhanced accuracy for high-viscosity solutions compared to conventional volumetric systems. Temperature control during filling operations helps maintain optimal viscosity conditions while preventing protein degradation.
Quality control testing for high-volume injectable formulations requires modified analytical procedures that accommodate unique formulation characteristics. Viscosity measurements become critical quality parameters that require standardized testing conditions and acceptance criteria. Particulate matter analysis may require specialized techniques to distinguish between acceptable excipient particles and problematic protein aggregates.
Regulatory Pathways and Clinical Considerations
Regulatory approval of high-volume injectable formulations requires comprehensive safety and efficacy data that address unique risks associated with large subcutaneous injection volumes. Clinical studies must demonstrate injection site tolerability, pharmacokinetic equivalence, and therapeutic efficacy compared to reference products or alternative dosing regimens.
Injection site tolerability studies evaluate patient responses to large-volume subcutaneous administration including pain, swelling, and local inflammation. These studies inform optimal injection techniques, volume limitations, and patient selection criteria for high-volume formulations. Advanced pain assessment techniques provide quantitative measurements of patient experience during and following injection procedures.
Pharmacokinetic studies compare absorption profiles of high-volume subcutaneous formulations to reference intravenous or intramuscular administration routes. These studies establish bioequivalence and identify any differences in absorption kinetics that may influence dosing strategies or therapeutic outcomes. Population pharmacokinetic modeling enables optimization of dosing regimens for diverse patient populations.
Safety monitoring programs track long-term outcomes following high-volume subcutaneous administration to identify delayed adverse effects and optimize clinical management protocols. These programs contribute to post-market surveillance databases that inform regulatory decision-making and clinical practice guidelines for high-volume injectable therapies.
Future Innovations and Technological Advancement
Nanotechnology applications in high-volume injectable formulations offer opportunities to enhance drug solubility and reduce viscosity through formation of stable nanoparticle dispersions. These systems can accommodate higher drug concentrations while maintaining favorable injection characteristics and potentially improving bioavailability through enhanced dissolution properties.
Smart formulation systems incorporate environmental sensors and responsive components that optimize injection characteristics based on physiological conditions. These systems can adjust viscosity or release rates in response to temperature, pH, or other physiological parameters to enhance therapeutic outcomes while maintaining injection tolerability.
Continuous manufacturing technologies enable real-time optimization of high-volume injectable formulations through process analytical technology and automated quality control systems. These approaches provide enhanced control over product quality while reducing manufacturing costs and improving supply chain flexibility for high-volume formulations.
Personalized medicine approaches utilize patient-specific dosing calculations and formulation modifications to optimize high-volume injectable therapy for individual patient needs. These strategies consider patient characteristics including body weight, disease severity, and pharmacogenetic factors to determine optimal dosing regimens and formulation requirements.
As pharmaceutical companies continue investing in high-volume injectable formulation technologies, the future promises increasingly sophisticated solutions that address current limitations while expanding therapeutic possibilities. Enhanced delivery systems, improved formulation strategies, and advanced manufacturing technologies will enable broader application of high-volume subcutaneous administration across diverse therapeutic areas, ultimately improving patient outcomes while reducing healthcare costs associated with traditional intravenous infusion procedures.