Advances in Lipid Nanoparticles for Gene and RNA-Based Therapies
The pharmaceutical landscape has experienced unprecedented transformation through the revolutionary development of lipid nanoparticles in RNA therapies, fundamentally altering how genetic medicines reach their therapeutic targets. These sophisticated delivery systems have evolved from experimental platforms into clinically validated technologies that enable the translation of genetic discoveries into life-saving treatments. The remarkable success of RNA-based COVID-19 vaccines demonstrated the transformative potential of lipid nanoparticles (LNPs) in delivering genetic material safely and effectively to human patients worldwide.
Lipid nanoparticles represent the culmination of decades of research in nanotechnology, molecular biology, and pharmaceutical engineering. These microscopic carriers, typically measuring 50-150 nanometers in diameter, provide protective environments that shield fragile RNA molecules from degradation while facilitating cellular uptake and cytoplasmic delivery. The sophisticated architecture of modern LNPs incorporates four distinct lipid components, each serving specific functions that collectively enable successful RNA therapeutics delivery.
Structural Engineering and Component Optimization
The foundation of effective RNA therapeutics delivery rests upon precise engineering of lipid nanoparticle formulations. Ionizable lipids serve as the cornerstone components, providing pH-dependent positive charges that facilitate RNA complexation during manufacturing while maintaining neutral surface charges at physiological pH to minimize toxicity. Advanced ionizable lipids such as ALC-0315 and SM-102, utilized in approved COVID-19 vaccines, demonstrate optimized pKa values that enhance endosomal escape while reducing systemic inflammation.
Phospholipid components provide structural integrity and membrane fusion capabilities essential for cellular uptake and intracellular delivery. Saturated phospholipids like distearoylphosphatidylcholine (DSPC) create stable bilayer structures, while unsaturated variants such as dioleoylphosphatidylethanolamine (DOPE) facilitate membrane destabilization and endosomal escape. The precise ratios of saturated and unsaturated phospholipids critically influence nanoparticle stability and delivery efficiency.
Cholesterol incorporation enhances membrane integrity and facilitates cellular interactions through native cholesterol recognition pathways. This naturally occurring lipid component improves nanoparticle stability during storage and circulation while promoting cellular uptake through receptor-mediated mechanisms. The cholesterol content typically comprises 20-40% of total lipid composition, with optimization required for specific RNA therapeutics applications.
Polyethylene glycol (PEG)-lipid conjugates provide surface functionalization that reduces protein adsorption and extends circulation times through stealth properties. These components create hydrophilic surface layers that minimize recognition by immune system components while enabling controlled biodistribution patterns. The selection of appropriate PEG molecular weights and surface densities critically influences pharmacokinetic profiles and targeting specificity.
Manufacturing Technologies and Quality Control
Microfluidic mixing represents the gold standard for lipid nanoparticle manufacturing, enabling precise control over particle formation and size distribution. These systems utilize rapid mixing of organic lipid solutions with aqueous RNA streams to create uniform nanoparticles through controlled precipitation. Advanced microfluidic devices incorporate multiple mixing stages and flow rate controls that optimize encapsulation efficiency while minimizing RNA degradation during processing.
Tangential flow filtration systems enable post-formation purification and concentration of lipid nanoparticle formulations. These processes remove unencapsulated RNA, excess lipids, and organic solvents while concentrating particles to desired therapeutic concentrations. Buffer exchange capabilities enable formulation optimization for stability and biocompatibility requirements specific to intended therapeutic applications.
Quality control analytical methods ensure consistent nanoparticle characteristics critical for therapeutic efficacy and safety. Dynamic light scattering measurements provide particle size and polydispersity determinations, while zeta potential analysis assesses surface charge characteristics. High-performance liquid chromatography enables quantification of individual lipid components and assessment of chemical stability throughout storage periods.
Encapsulation efficiency determinations utilize specialized assays that distinguish between encapsulated and free RNA molecules. These measurements directly correlate with therapeutic potency and enable optimization of manufacturing parameters. Advanced analytical techniques including cryo-electron microscopy provide detailed structural characterization of nanoparticle morphology and internal organization.
Cellular Uptake and Intracellular Trafficking
Lipid nanoparticles utilize multiple cellular uptake mechanisms to deliver RNA therapeutics to target cells effectively. Clathrin-mediated endocytosis represents the predominant uptake pathway, involving receptor-mediated recognition and vesicle formation. Macropinocytosis and caveolae-mediated endocytosis provide alternative uptake routes that may be cell-type specific or dependent upon nanoparticle characteristics.
Endosomal escape represents the critical rate-limiting step in RNA therapeutic delivery, requiring nanoparticle components to facilitate membrane disruption and cytoplasmic release. Ionizable lipids undergo protonation in acidic endosomal environments, creating electrostatic interactions with negatively charged endosomal membranes. This process destabilizes membrane integrity and enables RNA release into cytoplasmic compartments where translation machinery resides.
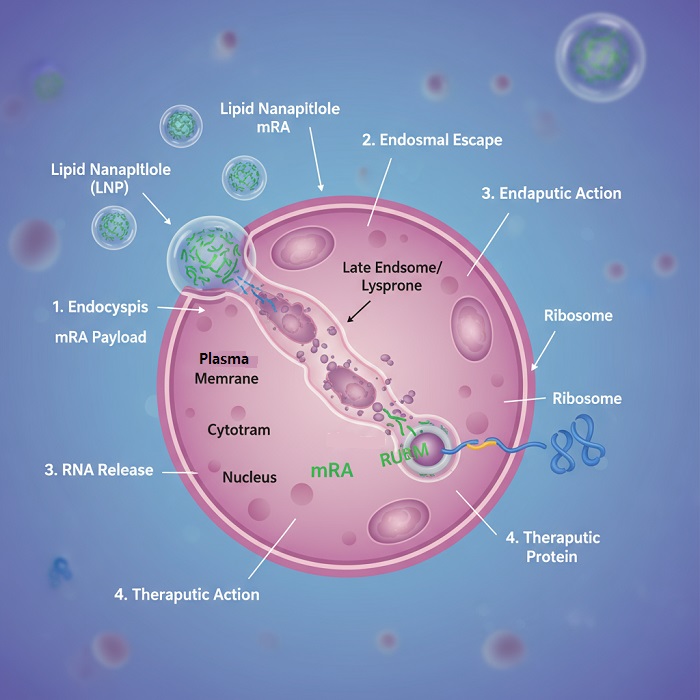
The efficiency of endosomal escape directly correlates with therapeutic efficacy, making this process a primary focus for nanoparticle optimization. Advanced formulations incorporate helper lipids and membrane-destabilizing components that enhance escape efficiency while minimizing cellular toxicity. Real-time monitoring techniques enable quantitative assessment of escape kinetics and optimization of formulation parameters.
Cytoplasmic RNA trafficking involves complex interactions with cellular machinery that influence therapeutic outcomes. Messenger RNA therapeutics must access ribosomal translation systems, while small interfering RNA molecules require incorporation into RNA-induced silencing complexes. Lipid nanoparticle formulations can be optimized to facilitate appropriate subcellular localization and therapeutic mechanism engagement.
Gene Therapy Applications and Therapeutic Targets
Gene replacement therapies utilize lipid nanoparticles to deliver corrective genetic material to cells with inherited deficiencies. These applications require precise targeting to affected cell populations while minimizing off-target effects in healthy tissues. Advanced formulations incorporate targeting ligands and tissue-specific delivery enhancements that improve therapeutic indices and reduce systemic toxicity.
Liver-directed gene therapies represent particularly successful applications of lipid nanoparticle technology, leveraging natural hepatic uptake patterns to achieve therapeutic concentrations. The FDA-approved siRNA therapeutic patisiran demonstrates clinical validation of this approach for treating transthyretin-mediated amyloidosis. Similar strategies show promise for treating metabolic disorders, genetic liver diseases, and hepatocellular carcinoma.
Muscle-targeted gene therapies utilize specialized lipid nanoparticle formulations designed to enhance uptake by skeletal and cardiac muscle tissues. These applications address muscular dystrophies, metabolic myopathies, and cardiac genetic disorders through delivery of corrective genes or therapeutic proteins. Advanced formulations incorporate muscle-specific targeting moieties that improve selectivity and therapeutic outcomes.
Central nervous system applications represent frontier areas for lipid nanoparticle-mediated gene therapy, requiring specialized formulations that cross blood-brain barriers or enable direct administration routes. These approaches show promise for treating neurodegenerative diseases, genetic brain disorders, and neurological cancers through delivery of neuroprotective genes and therapeutic proteins.
RNA Therapeutic Modalities and Mechanisms
Messenger RNA therapeutics enable transient protein expression for therapeutic purposes without permanent genetic modifications. These applications include protein replacement therapies for inherited deficiencies, vaccine antigens for infectious disease prevention, and therapeutic proteins for cancer treatment. Lipid nanoparticle delivery optimizes mRNA stability and translation efficiency while controlling expression duration and intensity.
Small interfering RNA therapeutics utilize RNA interference mechanisms to silence specific disease-causing genes through sequence-specific targeting. Lipid nanoparticle delivery protects siRNA molecules from nuclease degradation while facilitating cellular uptake and RISC complex loading. These approaches demonstrate therapeutic efficacy for treating genetic disorders, viral infections, and cancer through targeted gene silencing.
MicroRNA therapeutics modulate gene expression networks through regulation of multiple target genes simultaneously. These approaches enable complex therapeutic interventions that address disease pathways rather than individual targets. Lipid nanoparticle delivery systems optimize microRNA bioavailability and target engagement while minimizing off-target effects.
CRISPR-Cas9 gene editing systems utilize lipid nanoparticles to deliver both nuclease proteins and guide RNA components for precise genetic modifications. These applications enable correction of disease-causing mutations through targeted DNA editing while minimizing off-target effects. Advanced formulations optimize delivery of ribonucleoprotein complexes and enhance editing efficiency in target tissues.
Clinical Development and Regulatory Considerations
Clinical translation of lipid nanoparticle-based RNA therapeutics requires comprehensive safety and efficacy evaluations across diverse patient populations. Phase I studies establish safety profiles and dose-response relationships, while Phase II trials evaluate therapeutic efficacy in target patient populations. Advanced clinical trial designs incorporate biomarker assessments and pharmacokinetic analyses to optimize dosing regimens and treatment protocols.
Regulatory approval processes for RNA therapeutics emphasize product quality, manufacturing consistency, and clinical safety profiles. Agencies require extensive characterization of nanoparticle properties, including size distribution, encapsulation efficiency, and stability profiles. Manufacturing quality systems must demonstrate consistent production capabilities and appropriate analytical control strategies.
Immunogenicity assessments represent critical components of clinical development programs, evaluating both innate and adaptive immune responses to lipid nanoparticle components and RNA payloads. These studies inform dosing strategies and identify patient populations that may require modified treatment approaches. Advanced immunological monitoring techniques enable detection of subtle immune responses that could influence therapeutic outcomes.
Long-term safety monitoring programs track patient outcomes following RNA therapeutic administration to identify delayed effects and optimize clinical management strategies. These programs contribute to post-market surveillance databases that inform regulatory decision-making and clinical practice guidelines for emerging RNA therapeutics.
Future Directions and Technological Innovation
Targeted delivery systems represent the next generation of lipid nanoparticle technology, incorporating ligands and antibodies that direct therapeutic payloads to specific cell types and tissues. These approaches promise improved therapeutic indices through reduced off-target effects and enhanced efficacy in target tissues. Advanced targeting strategies utilize multiple recognition elements to achieve unprecedented delivery specificity.
Stimuli-responsive lipid nanoparticles incorporate components that respond to specific physiological conditions such as pH changes, enzymatic activity, or temperature variations. These systems enable controlled release of RNA therapeutics in response to disease-specific environmental cues, optimizing therapeutic timing and duration. Smart formulations can adapt their properties based on local tissue conditions to maximize therapeutic outcomes.
Combination therapies utilize lipid nanoparticles to deliver multiple therapeutic agents simultaneously, enabling synergistic treatment approaches that address complex disease pathways. These systems can co-deliver different RNA species or combine RNA therapeutics with small molecule drugs to achieve enhanced therapeutic effects. Advanced formulation strategies maintain individual component stability while optimizing combined therapeutic activity.
Personalized medicine applications leverage lipid nanoparticle technology to deliver patient-specific therapeutic agents based on individual genetic profiles and disease characteristics. These approaches utilize rapid RNA synthesis and formulation technologies to create customized treatments for rare diseases, personalized cancer therapies, and precision gene editing applications. As manufacturing technologies advance and costs decrease, personalized RNA therapeutics may become routine components of precision medicine practice, transforming how genetic diseases are treated and prevented across diverse patient populations worldwide.