Breakthroughs in Biologic Drug Formulation for Enhanced Stability
The pharmaceutical landscape has undergone remarkable transformation as biologic drug formulation stability takes center stage in modern therapeutic development. These complex molecular entities, ranging from monoclonal antibodies to gene therapies, represent the fastest-growing segment of pharmaceutical innovation, yet their inherent structural complexity poses unprecedented challenges for formulation scientists worldwide.
The foundation of successful biologic therapeutics rests upon achieving optimal biologic drug formulation stability throughout the entire product lifecycle. Unlike traditional small molecules, biologics demonstrate exponential sensitivity to environmental factors including temperature fluctuations, pH variations, and mechanical stress. This sensitivity creates a delicate balance between maintaining therapeutic efficacy and ensuring commercial viability through extended shelf life.

Advanced Formulation Technologies Revolutionizing Stability
Contemporary approaches to enhancing biologic formulation have evolved beyond conventional stabilization methods. Lyophilization techniques now incorporate novel cryoprotectants such as trehalose and sucrose, which form protective glassy matrices around protein structures during freeze-drying processes. These advanced formulations demonstrate remarkable improvements in biologic shelf life, often extending storage periods from 12 months to 36 months under optimal conditions.
The implementation of microfluidic mixing technologies represents another breakthrough in biologic drug formulation stability. These precision-engineered systems enable controlled particle formation and homogeneous distribution of stabilizing excipients, resulting in formulations with significantly improved consistency and reduced batch-to-batch variation. Recent pharmaceutical developments have shown that microfluidic-processed biologics exhibit 20-30% improved stability profiles compared to conventional mixing methods.
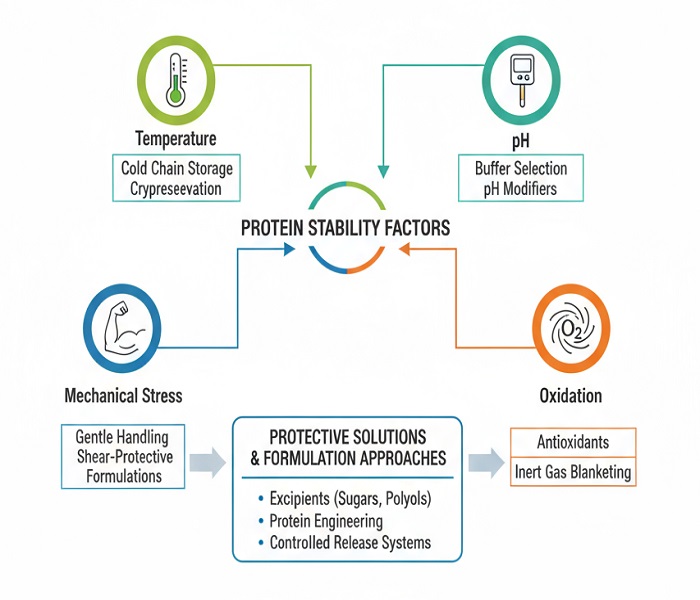
Innovative excipient systems have emerged as game-changers in addressing formulation challenges. Polysorbate-based surfactants prevent interfacial stress-induced aggregation, while histidine and phosphate buffer systems maintain optimal pH ranges throughout storage periods. The strategic incorporation of antioxidants, including methionine and ascorbic acid, provides crucial protection against oxidative degradation pathways that historically compromised biologic therapies.
Protein Engineering and Stability Enhancement
Modern biologic formulation strategies increasingly focus on molecular-level modifications to enhance intrinsic stability. PEGylation techniques involve covalent attachment of polyethylene glycol chains to protein surfaces, creating protective barriers that reduce immunogenicity while extending circulation times. These modifications represent sophisticated approaches to biologic drug formulation stability that address multiple challenges simultaneously.
The development of thermostable variants through directed evolution has revolutionized storage and distribution requirements for biologic therapies. These engineered proteins maintain structural integrity at elevated temperatures, potentially eliminating cold chain dependencies that traditionally limited global accessibility. Recent advances demonstrate thermostable biologics maintaining potency for extended periods at room temperature, representing paradigm shifts in pharmaceutical distribution strategies.
Site-specific modifications using click chemistry enable precise introduction of stabilizing elements without compromising therapeutic activity. These targeted approaches allow formulation scientists to address specific vulnerability points within protein structures, resulting in designer biologics with enhanced stability profiles tailored to particular therapeutic applications.
Analytical Technologies Driving Formulation Innovation
Real-time stability monitoring systems have transformed how pharmaceutical scientists approach biologic formulation development. Advanced spectroscopic techniques, including dynamic light scattering and differential scanning calorimetry, provide immediate feedback on formulation performance under stress conditions. These analytical capabilities enable rapid iteration cycles, accelerating the development of optimized biologic formulation strategies.
Predictive modeling approaches utilizing artificial intelligence algorithms can now forecast stability patterns based on molecular characteristics and formulation parameters. These computational tools reduce development timelines while improving success rates in achieving target biologic shelf life specifications. Machine learning models trained on extensive stability databases demonstrate remarkable accuracy in predicting long-term stability outcomes from accelerated testing data.
High-throughput stability screening platforms enable simultaneous evaluation of hundreds of formulation variants, dramatically expanding the scope of optimization studies. These automated systems can assess multiple stability parameters including aggregation propensity, chemical degradation rates, and potency retention across diverse storage conditions, providing comprehensive datasets for informed decision-making.
Novel Delivery Systems and Formulation Approaches
Nanoparticle-based delivery systems represent cutting-edge approaches to biologic drug formulation stability. Lipid nanoparticles, polymer microspheres, and inorganic carriers provide protective environments that shield sensitive biologics from degradation while enabling controlled release profiles. These sophisticated delivery systems address both stability concerns and therapeutic optimization simultaneously.
Self-assembling protein cages and virus-like particles offer innovative platforms for biologic encapsulation and protection. These biomimetic systems provide natural environments that maintain protein conformational stability while facilitating cellular uptake and targeted delivery. Recent developments in this field demonstrate remarkable improvements in both stability and therapeutic efficacy for encapsulated biologics.
Sustained-release formulation technologies enable extended therapeutic action while minimizing exposure to degradative conditions. Injectable depot systems utilizing biodegradable polymers can maintain therapeutic drug levels for weeks or months, reducing dosing frequency while ensuring consistent bioavailability. These advanced formulations represent significant improvements in patient compliance and treatment outcomes.
Regulatory Considerations and Market Impact
The regulatory landscape surrounding biologic formulation continues evolving to accommodate innovative stability enhancement strategies. Regulatory agencies increasingly recognize the value of advanced formulation technologies in improving patient access to life-saving therapies. Streamlined approval pathways for formulation improvements enable faster implementation of breakthrough stabilization approaches.
Quality by design principles have become integral to biologic formulation development, emphasizing systematic understanding of formulation variables and their impact on product quality. This approach ensures robust biologic drug formulation stability throughout commercial manufacturing while providing flexibility for continuous improvement initiatives.
The economic implications of enhanced biologic stability extend far beyond reduced manufacturing costs. Improved shelf life enables expanded global distribution networks, particularly in regions with limited cold storage infrastructure. These improvements democratize access to advanced biologic therapies while creating new market opportunities for pharmaceutical companies.
Future Directions and Emerging Technologies
Personalized formulation approaches represent the next frontier in biologic drug development. Patient-specific factors including genetic variations, disease characteristics, and concurrent medications increasingly influence formulation strategies. These personalized approaches promise improved therapeutic outcomes through optimized biologic formulation stability tailored to individual patient needs.
Continuous manufacturing technologies offer unprecedented control over formulation processes, enabling real-time adjustment of parameters to optimize biologic stability. These advanced manufacturing systems provide consistent product quality while reducing production costs and environmental impact through improved efficiency.
Smart packaging systems incorporating environmental sensors and communication technologies enable real-time monitoring of product integrity throughout distribution chains. These innovations provide comprehensive visibility into storage conditions and product status, ensuring maintained biologic shelf life from manufacturing to patient administration.
The convergence of biotechnology, materials science, and digital technologies continues driving innovation in biologic formulation development. Emerging approaches including 3D printing of personalized dosage forms, responsive packaging systems, and blockchain-enabled supply chain monitoring represent transformative opportunities for enhancing biologic drug formulation stability while improving patient outcomes worldwide.
As the pharmaceutical industry continues embracing these technological advances, the future of biologic therapeutics appears increasingly promising. Enhanced stability profiles will enable expanded therapeutic applications, improved global accessibility, and ultimately better patient outcomes across diverse medical conditions requiring advanced biologic therapies.























