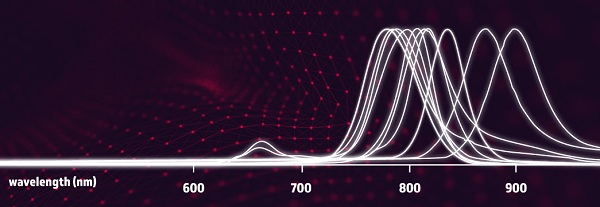
Biotium, a leading supplier and inventor of fluorescent probes for life science research, announces the release of infrared CF®850 and CF®870 dyes. These novel long wavelength dyes are the first commercially available fluorescent dyes with emission maxima greater than 850 nm, allowing researchers to further investigate the “final frontier” of infrared wavelengths for life science research applications. Due to their excellent brightness, aqueous solubility, and exceptional infrared properties, CF®850 and CF®870 represent a true breakthrough in synthetic fluorescent probes.
Infrared (IR) and near-infrared (near-IR) probes offer several advantages over probes in the visible light region. Their superior sensitivity, linearity, tissue penetration, and minimal autofluorescence background are beneficial for in vivo imaging, western blotting, and microscopy. In addition, the availability of probes that extend further into IR wavelengths enables greater multiplexing options for flow cytometry. Recently, the benefits of long wavelength fluorescence have spurred the development of flow cytometers and imaging instrumentation equipped with near-IR laser diodes and detection systems. Biotium’s CF®850 and CF®870 dyes aim to accommodate research that takes advantage of these hardware advancements by offering long wavelength emission beyond any commercially available IR probe.
CF®850 and CF®870 are optimally designed for the 808 nm laser but may also be excited by the 785 nm laser. They are currently available as stable amine-reactive TFP (tetrafluorophenyl) esters but will be available soon as labels for commonly used antibodies, bioconjugates, and with Biotium’s easy-to-use Mix-n-Stain™ Antibody Labeling Kits.
CF®850 and CF®870 are part of Biotium’s family of patented pegylated CF® Dyes and adds to Biotium’s growing list of novel long wavelength dyes for life science research. Biotium’s IR and near-IR dyes also include CF®700, CF®750, CF®770, CF®790, CF®800, CF®820, and the recently released flow cytometry tandem dyes APC-CF®750T and APC-CF®790T.
About Biotium
Biotium is a leading life science reagent manufacturer and supplier devoted to providing high-quality and innovative fluorescent tools that fuel scientific discovery. Our collaborative team of experienced chemists and biologists, who are at the forefront of fluorescent dye design, apply chemistry-based principles toward producing solutions for unmet challenges in life science and medical research. Since its founding in 2001, Biotium has developed over 30 patented technologies that have been licensed out to leading life science technology companies worldwide.