PatientSource has announced that it will be offering an adapted version of its Electronic Patient Record (EPR) software to healthcare organisations across the globe, completely free of charge with minimal cloud hosting costs, to help combat the Coronavirus pandemic.
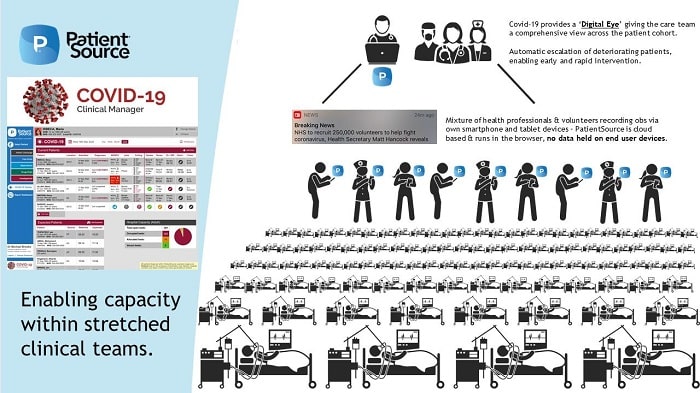
With the priority to ease the burden on healthcare services now reaching critical levels, PatientSource is offering a ‘slimmed down’ version of its software, which can be installed and ready to go within as little as an hour, in a move to keep a ‘digital eye’ over the ever-increasing number of COVID-19 patients.
Founded by a practising NHS doctor and recognised as a ‘leading innovator’ by the Department of International Trade, PatientSource offers a cloud-based interoperable patient record system with an intuitive interface, designed for use on the frontline of care. The clinician-designed solution works cross-platform on any device with a web browser – desktops, laptops, tablets and smartphones. The company has an international footprint, with clients in the NHS and private sector.
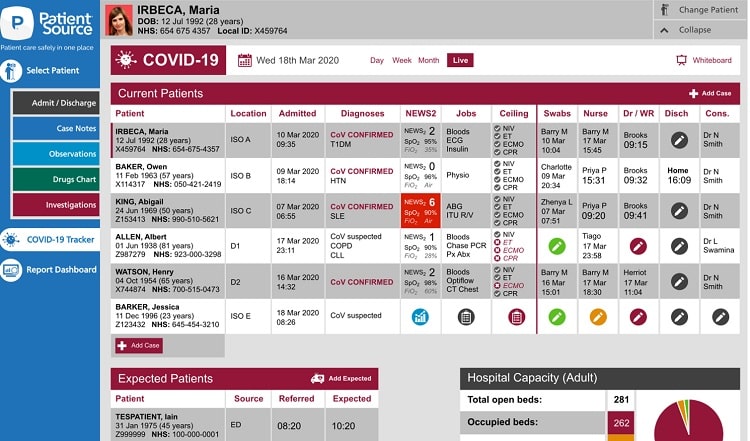
The adapted version of the PatientSource software will feature a cloud-based electronic observation module complete with patient trackers and ward whiteboard tools that are attuned to COVID-19 patients, and will be able to quickly identify the most critical, frail or deteriorating patients in a challenging setting.
Dr Michael Brooks, Chief Medical Officer and Co-Founder of PatientSource, said: “COVID-19 is a virus that spreads quickly and causes an appreciable minority of people to need hospital care. Put those two figures together, and you get a virus that will easily overwhelm any country’s healthcare system. Hospitals across the globe are going to be saturated and will struggle to cope.
“PatientSource brings case tracking, clinical noting, vital signs, test requests and results, team messaging, and prescribing together in one place. A doctor or nurse can walk onto a ward with just one tablet and access everything they need.”
“Our PatientSource COVID-19 tracker will show you which affected patients are in your hospital or ward, what their latest vitals are, the plans for escalation, and who the expected incoming cases are in real-time. This allows you to identify the patients who need oxygen bays and the patients who need critical care input, allowing you to allocate limited resources to those who need them quickly.”
Recent announcements from the Department of Health and Social Care have revealed that they will soon call upon private hospitals and possibly requisition hotels to help deal with the ever-growing number of NHS patients, meaning that resources are to be spread thinly and widely. PatientSource hopes that the capabilities of its EPR solution in providing digital oversight over a rapidly expanding patient cohort will support overstretched medical services both in the UK and across the globe.
Its experience of working across both sectors and the interoperable capabilities of its EPR solution will help providers to maximise the efficiency of their available resources by applying them appropriately to the most critical patients.
Dr Brooks added: “As an Emergency Department Clinician I acutely understand the challenges faced by my peers in caring for large numbers of patients with limited staff resources. This offer aligns to my founding ethos for PatientSource of better patient care; I hope we can help.”
About PatientSource
PatientSource provides an Electronic Medical Record (EMR) solution which is disrupting the healthcare market. The company has a global footprint and track record of success working with the NHS and private sector. Founded by practising clinicians, we are empowering healthcare organisations across the world to move away from paper-based records. Unique in its offering to the global healthcare market, this affordable EMR promises greater user experience and the safer, more efficient delivery of care.