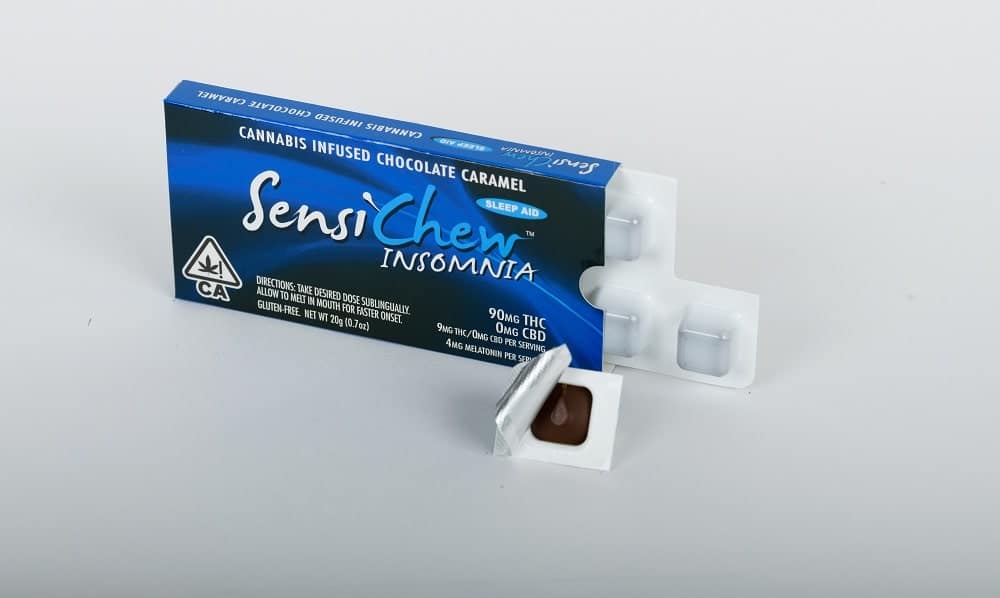
Sensi Products, the licensed cannabis manufacturer of Sensi Chew, has introduced new pharma-grade and child-resistant packaging.
The new California compliant packaging, which features an informational insert to improve the user experience, has been designed for cannabis infused chocolate caramels.
The new packaging and automated manufacturing process will help the company to meet the growing market demand for its cannabis edibles.
Available in more than 240 licensed dispensaries and delivery services across California, Sensi Chews are provided in 90mg pack with 10-9mg individual doses.
The company is providing a family of nine products under three specific categories.
Classic is comprised of Sativa, Indica, Hybrid, while Specialty includes added formulations for insomnia, energy, and sexual enhancement. CBD is comprised of CBD-only, 1:1 CBD to THC, and CBD for Insomnia.
Sensi Products CSMO Lisa Tollner said: “Since its inception Sensi Chew has focused on helping users select a product based on time of day use and will continue to use our ‘Daytime. Nighttime. Anytime’ product segmentation to assist consumers in knowing when to use which chew. All products are color-coded to help users as well as budtenders to easily identify each product.”
Sensi Chew Insomnia is a major cannabis sleep-aid product on the market, said the company.
The company has made an investment in research on sleep, pain, and anxiety, and is planning to launch focused reports on the findings.
Sensi Products CEO Edic Sliva said: “Our goal is to bring Sensi Chew Insomnia to the millions of people who struggle to sleep each night.
“Sensi Chew Insomnia can replace the harmful side-effects of traditional sleep aids to give users a healthy alternative and the deep restful sleep they deserve.”
Sensi Products was established by Edic Sliva and Lisa Tollner in 2013. Edic Sliva is also the founder of Microlux that offers electronics, design, and manufacturing services for medical devices, consumer goods, automotive and military products.
Lisa Tollner established Cintara Corp, which is a full-service brand marketing firm that provides services to firms in high tech, healthcare, consumer goods, and hospitality industries.