Daiichi Sankyo Company, Limited announced that it has launched in Japan an injection for cancer pain treatment: NaruveinⓇ Injection 2 mg • 20 mg.
Hydromorphone hydrochloride is an opium-based narcotic analgesic that has been sold for more than 80 years outside Japan and positioned as the standard drug for pain management in the World Health Organization (WHO) guidelines for cancer pain treatment.
Joining the currently marketed extended-release formulation, NarususⓇ Tablets, and immediate-release formulation, NarurapidⓇ Tablets, the injection formulation, NaruveinⓇ Injection, is a new addition to Daiichi Sankyo’s analgesic line-up to help meet the diverse needs of patients and health care professionals in Japan.
Hydromorphone hydrochloride is one of the agents publicly offered for development by the Review Committee on Unapproved Drugs and Indications with High Medical Needs*. Daiichi Sankyo decided to develop the drug in 2012, and received a grant from the Pharmaceutical Development Support Center for its development.
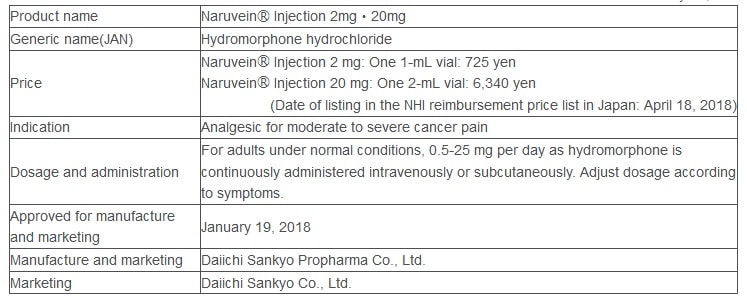
Product Outline
Daiichi Sankyo is committed to making unapproved and off-label drugs with high medical needs available to the patients who are waiting for them.
* A committee held by Japan’s Ministry of Health, Labour and Welfare that aims to promote the development of drugs and indications not yet approved in Japan, but currently available in Europe and the U.S.
About Daiichi Sankyo Opioid Analgesics
Daiichi Sankyo contributes to the total care for the life of patients with cancer by providing opioid analgesics. A diverse line-up of narcotic analgesic drugs supports patients living with cancer by relieving pain and improving QOL. In Japan, in addition to the hydromorphone chloride preparations, NarususⓇ Tablets (extended-release formulation), NarurapidⓇ Tablets (immediate-release formulation), and NaruveinⓇ Injection (injection formulation), Daiichi Sankyo also markets the oxycodone hydrochloride preparations, Oxycodone Extended Release Tablets Daiichi Sankyo