Introducing a new disease-modifying therapy, from research through to launch, is an extremely complex process, with lengthy and expensive drug development cycles. While clinical trial design has improved, the failure rate is still significant, especially in areas such as neurology. In fact, it is estimated that from 2002 to 2012, approximately 99.6% of drug trials in Alzheimer’s disease (AD) failed.1 A recent study shows that, under the current conditions, only drugs currently in late Phase 1 or later will have a chance of being approved by 2025.2
One reason for this high failure rate is the heterogeneity of the disease, and how it manifests in different patients, including those who volunteer and are selected to participate in clinical trials. The result is that pharma companies have a mixed population of clinical trial participants, making it difficult to demonstrate drug efficacy.
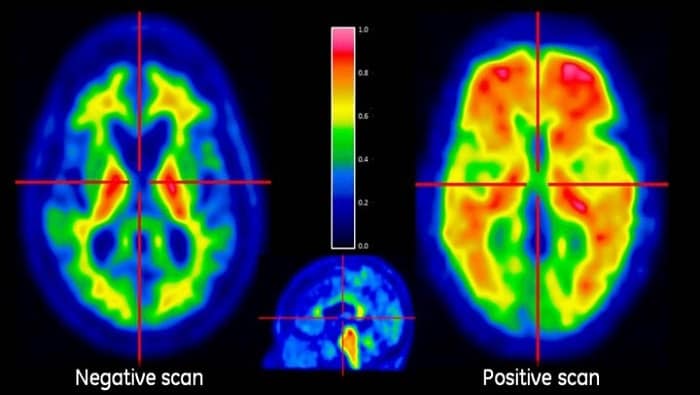
Since accumulation of beta-amyloid plaque in the brain is a requirement for the diagnosis of AD, pharmaceutical companies typically recruit patients who have a significant buildup of abnormal proteins in the brain that form beta-amyloid plaques. Beta-amyloid plaque deposition may begin as early as 10 to 15 years before any cognitive impairment occurs, and a disease process begins that is specific to each individual patient. This means that even though patients have abundant beta-amyloid plaques in the brain, they may not develop AD at the same rate or progress beyond mild cognitive impairment (MCI), a condition that precedes AD and that causes a slight decline in cognitive abilities but doesn’t always lead to dementia.
In order to potentially alter the path of AD progression, pharmaceutical companies studying disease- modifying therapies should conduct clinical trials in subjects at earlier stages of the disease, rather than later when neurodegeneration has already led to overt cognitive and functional decline.
Pharmaceutical companies are, therefore, incorporating and evaluating new clinical trial enrichment strategies to help them identify and include patients who are in the early stages of AD (before neurodegeneration has started) and in those who are declining more rapidly than others in the hope that any effect of an investigational drug will be detected.
A new predictive analytics tool is now under development to facilitate the identification and selection of appropriate subjects for these clinical trials. This digital biomarker app, developed by GE Healthcare imaging and data scientists, could help pharma companies improve the selection process by choosing patients with a high probability of having beta-amyloid plaques in the brain, and in those who may progress from MCI to AD faster than others. Hopefully, this could increase the rate at which effective disease-modifying therapies become available.
A Neurology scan taken using GE’s PET/MR technology.
With the new digital biomarker app, it is possible to combine patient data from imaging exams with clinical information such as psychometric test scores, demographics, and genetic testing, helping to predict disease progression with more confidence than has been possible to date. The Web-based app generates a “probability score” to show how likely a patient is to have beta-amyloid plaques in the brain and progress from MCI to AD within three years.
The tool, which was recently presented at the 10th Clinical Trials on Alzheimer’s Disease (CTAD) in Boston, is currently able to predict disease progression with 86% accuracy and 92% specificity, an improvement of 24% on existing methods. With preliminary data showing how this approach could improve the efficiency of recruitment for a trial, GE Healthcare scientists are now focusing on the acquisition of more data to conduct beta testing and further improve the model’s predictive performance.