BD , a leading global medical technology company, announced the commercial release of the BD™ AbSeq assay to analyze protein expression at the single-cell level using high-throughput sequencing. The assay brings together high-quality antibodies from the BD Pharmingen™ portfolio with oligonucleotides, allowing researchers to perform single-cell protein analysis on the BD Rhapsody™ single-cell analysis system.
When used together, these research tools enable researchers to simultaneously analyze RNA and proteins in thousands of individual cells and develop a more complete picture of the role genes and proteins play in biological systems.
The product leverages 40 years of BD leadership in immunology research. Initially available with more than 100 different human antibody-oligonucleotide combinations, over time BD will rapidly expand the portfolio to utilize its vast library of flow cytometry-proven human and mouse antibody clones, helping researchers to advance understanding of complex diseases. Customers can also design their own custom conjugates.
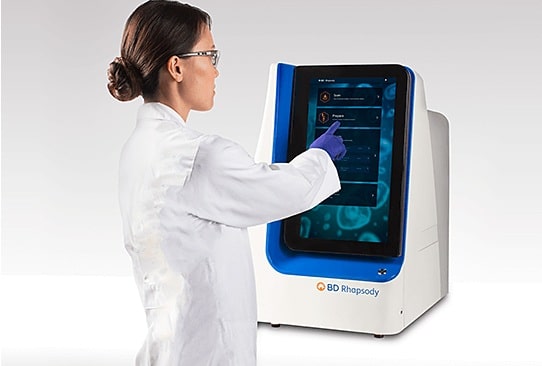
To advance knowledge of the immune system, BD empowers immunology researchers with a range of tools for multi-omics analysis. The launch of the BD™ AbSeq assay is the latest innovation in BD’s single cell multi-omics portfolio. Over the last year, BD has built on its BD Rhapsody™ single-cell analysis system, a complete system of reagents, instruments, software, and targeted gene panels, to offer additional single-cell analysis capabilities.
These include the BD Rhapsody™ Express system and the BD™ single-cell multiplexing kit. These new offerings provide targeted RNA-seq options and enable sample pooling to improve data quality and reduce time to discovery.
“BD is committed to single-cell research and delivering easy-to-use tools with a low barrier to entry,” said John Ledek, worldwide president of BD Biosciences. “Single-cell analysis tools like the BD Rhapsody system and the BD™ AbSeq assay will maximize our customers’ ability to scrutinize cells of interest and power rapid advancements in immunology including drug treatment response, cell therapy, and beyond.”
More information on the new BD™ AbSeq assay is available at bd.com/genomics.
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical process for health care providers. BD and its 65,000 employees have a passion and commitment to help improve patient outcomes, improve the safety and efficiency of clinicians’ care delivery process, enable laboratory scientists to better diagnose disease and advance researchers’ capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiencies, improve safety and expand access to health care. In 2017, BD welcomed C. R. Bard and its products into the BD family. For more information on BD, please visit bd.com.
For more information on BD, please visit bd.com.